
A few weeks ago, we were reading a 5 Acres and a Dream blog post about making homemade leavening from wood ashes (i.e., from potassium carbonate, K
2CO
3), and a reader in the comments section asked if calcium carbonate (CaCO
3) from eggshells, which also reacts with acid to release CO
2 gas (reaction below), could be used as a leavening agent. We had been wondering the same thing for quite a while, and the realization that other folks were wondering the same thing provided the motivation we needed to finally get up and do some experiments.
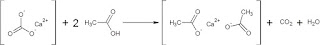 |
| Calcium carbonate (CaCO3) reacts with acetic acid in vinegar to make calcium acetate, carbon dioxide gas (CO2), and water (H2O). |
First, some eggshell chemistry. Eggshells are about 95% CaCO
3, but the CaCO
3 is bound in a matrix of protein, with a proteinaceous membrane also attached. Thus, one might expect that eggshells would make a better leavening agent if the CaCO
3 could be isolated from the protein (and/or ground very finely) so that it would be more accessible to the acid during baking. The question is, how to get rid of the protein? Well have to either dissolve the protein away from the CaCO
3 or dissolve the CaCO
3 away from the protein and then regenerate it somehow. Today well try the former.
Theres quite a bit of precedent for dissolving away the eggshell protein (or at least, most of it) with a strong base, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), but specific recipes are hard to come by. Several articles refer to this original gem, the most useful being this one, which allows us to deduce that those guys boiled their eggshells in a 2.5 wt% NaOH solution for 5 minutes, which easily removed the membrane and part of the protein matrix. They then increased the lye concentration to 10 wt% and boiled for a long time, finding that all the protein that could be removed was gone by about 7 hours. They didnt give a lye-to-eggshell ratio, though. Additionally, this patent references another patent (we couldnt track down the original) claiming that boiling eggshells in 3 wt% NaOH would reduce the protein content of the shells to
< 0.1%, although the boiling time and lye-to-eggshell ratio wasnt specified.
A protein content of
< 0.1 wt% sounds good enough to us, so we decided to follow that route most closely. Having to guess on the time and lye-to-eggshell ratio, we decided that if we had to boil for more than half an hour and use more than a 1:1 ratio, that it wouldnt be worth our trouble. (In that case, wed just use Leighs ash-based leavening instead!) Alright, experiment planned; lets do this!
 |
| Heres our recipe: 15 g NaOH, dissolved in 500 g tap water, with 15 g coarse-ground eggshells (1-2 mm particles) added. Boiled for 30 min. Wear safety glasses and gloves until everything is neutralized later on (see below). |
 |
| As the mixture simmered, the lye water turned a cloudy yellow. A good sign that were dissolving protein. |
 |
| After boiling, we poured the liquid through a coffee filter (supported by a polypropylene funnel) into a quart jar. The eggshells dont look that much different than before, except maybe slightly darker. The pigment (they were brown shells) is still there. The coffee filter is really slow, so something like an old t-shirt or terrycloth towel might be better. |
 |
| The filtrate is still highly caustic, so be careful with it! We wanted to neutralize it before doing anything else, so we added a couple tablespoons of our good ol red cabbage pH indicator, causing the filtrate to go from yellow to slightly-darker-yellow. Note if youre following along at home--dumping the filtrate down the drain without neutralizing might kill some of your friendly septic system bugs, so please neutralize! |
 |
| Then we added vinegar until it turned green, then blue, then finally purple, indicating a neutral pH. (We had to add a few more tablespoons of pH indicator as it got more and more dilute, because the color changes started to get hard to see.) Now it can go down the drain or into the compost. |
 |
| The next step is to repeatedly rinse the boiled eggshells to wash all the lye off. These are the rinses (plus pH indicator), showing steadily decreasing alkalinity. After the fourth rinse (which was with vinegar), the filtrate is neutral, the eggshells should be substantially free of lye (and hopefully protein!), and were good to move on (and take off our safety glasses and gloves). We also neutralized the second and third filtrates with vinegar, too. Safety note: working with lye on something youre planning to eat has the potential to cause some serious damage if you dont neutralize properly. Be careful and only do this if youre comfortable with the chemistry! Also, make sure youre using pure lye, and not some cleaner that has lye combined with other chemicals. |
 |
| Drying the lye-boiled eggshells makes them easier to work with. In the oven at 300 °F for 15-20 min ought to do the trick! |
 |
| Time to make some experimental biscuits! Five sets of three biscuits each. Recipe per set: 0.5 cups all purpose flour, 0.25 teaspoon leavening, 0.125 (1/8) teaspoon salt, 1 tablespoon butter (in the bowls), 1 teaspoon apple cider vinegar plus milk (2%) to bring the volume up to 0.25 cups to make a faux buttermilk (in the glasses). We processed the bowl contents in a food processor for about 5 seconds to cut in the butter, then added the "buttermilk" and processed for another 5 seconds to mix everything up, scooped the dough/batter into drop biscuits and baked at 400 °F for about 20 min. The five sets differ only in their leavening: no leavening, lye-boiled coarse-ground eggshells, coarse-ground eggshells, fine-ground eggshells, and baking soda. |
 |
| After baking, there a clear difference between the No Leavening control and the rest, but also between the baking soda and the rest. Between the eggshell sets, the lye-boiled and the finely-ground are about equal, and slightly more risen than the coarse-ground. However, all the eggshell sets are very close to each other, and closer to the control than to the baking soda. Also, we didnt have enough room for all fifteen biscuits on this sheet, so we baked the third biscuit of the last three sets separately. Their appearance was consistent with the biscuits here. Hooray for reproducibility! |
 |
| The textures are consistent with the appearance, but its hard to tell from the photos. Also, the biscuits other than the baking soda set werent cooked all the way through after 20 min. We put them back in the oven; after another 15 minutes they were no longer doughy, but they didnt rise any more. The flavor of all the sets is decent, so the control set and the eggshell sets would make decent dumplings if youre into that sort of thing. Overall, the conclusion from these experiments is that the eggshells provide some leavening effect, but not much. For us, the ground eggshells dont really get the job done, and its definitely not worth the effort of boiling them in lye water to dissolve off the protein. |
But were not done with these experiments yet! Stay tuned for Part 2, where we dissolve and regenerate the calcium carbonate part, and see how that works as leavening!













